Evaluating Dissected NICS with NBO Program
General information
This blog is a memo for calculation NICS values from π-orbital contributions, so-called “dissected NICS values”. NBO 3.1, which is a built-in module in Gaussian 09/16 is not available for dissected NICS calculation. At least NBO 5.0+ is necessary.
Computation
This memo is based on the paper “Which NICS Aromaticity Index for Planar π Ring is Best?”. For more detail informations, please refer to the paper and supporting information.
Input
A general input file for evaluating dissected NICS values of biphenyl with NBO program is shown below, all input and output files of this example could be download from here.
#p nmr rb3lyp/6-31+g(d) iop(10/46=1) pop=nbo7read
NICSinput//Created_by_py.Aroma
0 1
H 1.90900000 -0.93500000 0.91600000
C 1.07900000 -0.51700000 1.45200000
C -1.07900000 0.51700000 2.83500000
C 0.00000000 0.00000000 0.74500000
C 1.07900000 -0.51700000 2.83500000
C 0.00000000 0.00000000 3.53100000
C -1.07900000 0.51700000 1.45200000
H 1.91500000 -0.92500000 3.36700000
H 0.00000000 0.00000000 4.60300000
H -1.90900000 0.93500000 0.91600000
H -1.91500000 0.92500000 3.36700000
C 0.00000000 0.00000000 -0.74500000
C 0.00000000 0.00000000 -3.53100000
C 1.07900000 0.51700000 -1.45200000
C -1.07900000 -0.51700000 -1.45200000
C -1.07900000 -0.51700000 -2.83500000
C 1.07900000 0.51700000 -2.83500000
H 1.90900000 0.93500000 -0.91600000
H -1.90900000 -0.93500000 -0.91600000
H -1.91500000 -0.92500000 -3.36700000
H 1.91500000 0.92500000 -3.36700000
H 0.00000000 0.00000000 -4.60300000
Bq 0.432106 0.901823 2.138333
Bq -0.432106 -0.901823 2.138333
Bq 0.432106 -0.901823 -2.145000
Bq -0.432106 0.901823 -2.145000
Bq 0.000000 0.000000 2.145000
$NBO NCS <XYZ MO> $END
Output
An example of output is shown below. Find the following table in the output file:
Principal components of the tensor (ppm) for atom gh( 26):
Canonical MO contributions
This tensor is non-symmetric. The antisymmetric part will be printed.
Principal components Antisymmetric part
MO 11 22 33 12 13 23 CSA ISO
=================================================================================
1. -0.12 0.04 -0.11 0.04 0.03 -0.06 -0.07 -0.06
2. 0.94 0.02 0.51 -0.15 -0.15 0.36 0.02 0.49
3. 0.54 0.04 1.87 0.01 -0.02 -0.21 1.57 0.82
4. -0.23 -0.04 1.01 0.00 -0.22 -0.15 1.15 0.25
5. 0.22 0.04 0.89 -0.10 -0.21 -0.46 0.75 0.38
6. -0.06 -0.20 0.82 0.07 -0.02 -0.23 0.95 0.19
7. 0.35 0.02 1.76 -0.02 -0.07 -0.65 1.58 0.71
8. -0.02 0.01 1.63 0.01 -0.22 -0.58 1.64 0.54
9. 0.15 0.35 0.43 0.18 0.07 -0.34 0.17 0.31
10. 0.00 -0.12 0.28 -0.24 0.05 -0.13 0.34 0.05
11. 0.19 0.13 -1.90 0.19 0.17 -0.12 -2.07 -0.53
12. 0.09 -0.03 -0.18 -0.04 -0.18 -0.21 -0.21 -0.04
13. -1.71 -0.81 2.41 0.11 -1.50 -2.05 3.67 -0.04
14. 0.93 -0.66 5.42 0.04 -1.61 -4.67 5.29 1.90
15. -0.20 0.56 5.55 0.02 -0.97 -1.87 5.37 1.97
16. 1.25 -0.03 6.01 -0.70 -0.42 0.37 5.40 2.41
17. 1.05 0.92 3.55 0.18 0.42 -1.06 2.56 1.84
18. -1.07 0.36 -1.57 -0.18 0.32 -2.00 -1.22 -0.76
19. 1.16 0.78 2.51 -0.05 -0.08 -0.57 1.53 1.48
20. 1.55 0.91 2.59 0.51 0.12 -0.34 1.37 1.68
21. 0.65 0.67 2.53 -0.60 -0.22 -0.35 1.88 1.28
22. 0.65 1.00 2.22 -0.10 -0.41 -0.98 1.39 1.29
23. 0.36 0.28 2.70 0.25 -0.67 -3.77 2.38 1.12
24. -0.21 -0.25 3.89 -0.06 -0.55 -2.25 4.13 1.14
25. -0.27 -0.13 0.78 0.13 -0.12 0.14 0.99 0.13
26. -2.15 -1.40 2.49 0.27 -0.26 1.89 4.27 -0.35
27. 0.13 0.02 1.02 -0.04 -0.59 -1.27 0.95 0.39
28. -1.91 -1.11 -0.48 -0.40 0.55 2.11 1.03 -1.17
29. -0.16 -0.46 -2.95 0.35 1.23 4.44 -2.64 -1.19
30. -0.24 -1.07 -3.56 0.16 1.32 5.19 -2.90 -1.62
31. 0.00 0.46 -3.39 0.15 -0.32 -0.91 -3.62 -0.98
32. 3.17 1.94 3.43 -0.25 -0.41 -1.02 0.88 2.85
33. -1.20 -0.26 -12.71 -0.07 0.75 2.42 -11.98 -4.72
34. 3.47 2.77 5.07 -0.06 -0.80 -1.51 1.95 3.77
35. -1.48 0.09 -8.69 -0.30 0.87 3.42 -7.99 -3.36
36. -0.56 -1.01 -7.56 0.08 1.07 2.92 -6.77 -3.04
37. -0.97 -0.08 -6.94 0.11 0.27 1.12 -6.42 -2.66
38. -2.65 0.58 6.40 0.23 1.69 -0.88 7.43 1.45
39. 0.88 -2.65 4.07 -0.02 0.37 1.72 4.95 0.77
40. -0.59 -2.23 4.88 0.43 -0.08 2.04 6.29 0.68
41. -2.09 2.02 1.22 0.18 0.66 0.64 1.26 0.38
---------------------------------------------------------------------------------
Total -0.19 1.47 27.87 0.31 -0.13 0.13 27.23 9.72
---------------------------------------------------------------------------------
In this case, the π-orbitals could be identified to MO 32, 34, 38, 39 40 and 41 by examining the MO structure. So, we could calculated the NICS values:
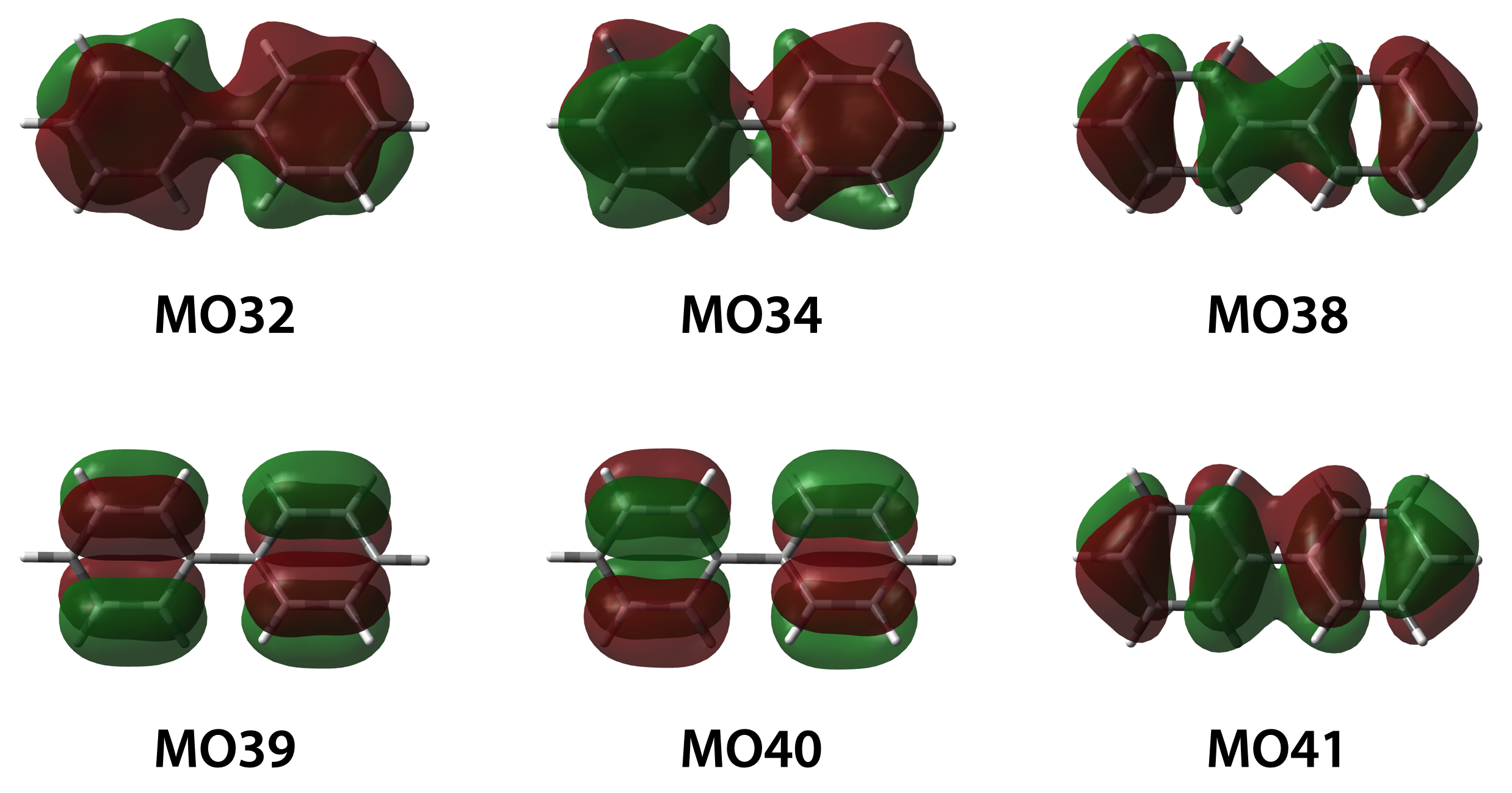
- NICS(zz) = -27,87 ppm, showed in “Principal components - 33” column.
- NICS(iso) = -9.72 ppm, showed in “ISO” column.
- NICS(π,zz) = -(3.43 + 5.07 + 6.40 + 4.07 + 4.88 + 1.22) = -25.07 ppm.
- NICS(π,iso) = -(2.85 + 3.77 + 1.45 + 0.77 + 0.68 + 0.38) = -9.9 ppm.
Processing output with py.Aroma
The upcoming py.Aroma v2.0 will add the support for NICS calculation with NBO program. By inputting the π-orbitals number, the NICS(zz), NICS(iso), NICS(π,zz) and NICS(π,iso) values of each ghost atom would be summarized in table.
Atom NICS(zz) NICS(iso) NICS(π,zz) NICS(π,iso)
==================================================================
Bq( 23) -27.87 -9.71 -25.07 -9.9
Bq( 24) -27.87 -9.71 -25.07 -9.9
Bq( 25) -27.87 -9.72 -25.07 -9.9
Bq( 26) -27.87 -9.72 -25.07 -9.9
Bq( 27) -12.12 -7.74 -30.05 -22.12
Enjoy Reading This Article?
Here are some more articles you might like to read next: