publications in 2021
all / 2025 / 2024 / 2023 / 2022 / 2021 / 2020 / 2019 / 2018
- Review
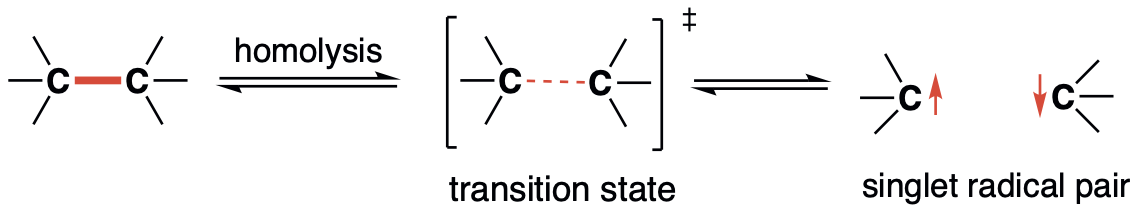 New Insights into Bond Homolysis Process and Discovery of Novel Bonding System (C–π–C) by Generating Long-lived Singlet DiradicalsM. Abe*, Z. Wang, and R. AkisakaAsiaChem, 2021, 2, 32–41.
New Insights into Bond Homolysis Process and Discovery of Novel Bonding System (C–π–C) by Generating Long-lived Singlet DiradicalsM. Abe*, Z. Wang, and R. AkisakaAsiaChem, 2021, 2, 32–41.In recent years, low-valent chemical species such as radicals and carbenes, which have been recognized as short-lived intermediates, have been isolated by appropriate molecular design, and their chemical properties have been investigated in detail experimentally. In particular, the discovery of isolable carbenes, which are now widely used as indispensable ligands in coordination chemistry and synthetic organic chemistry, has enabled the development of novel highly active catalysts.
@article{abe2021new, title = {New Insights into Bond Homolysis Process and Discovery of Novel Bonding System (C–π–C) by Generating Long-lived Singlet Diradicals}, author = {Abe, M. and Wang, Z. and Akisaka, R.}, journal = {AsiaChem}, volume = {2}, issue = {1}, pages = {32--41}, year = {2021}, month = dec, publisher = {ics}, doi = {10.51167/acm00020.51167/acm00021}, url = {https://doi.org/10.51167/acm00020.51167/acm00021}, dimensions = {true}, tab = {review} } - Review
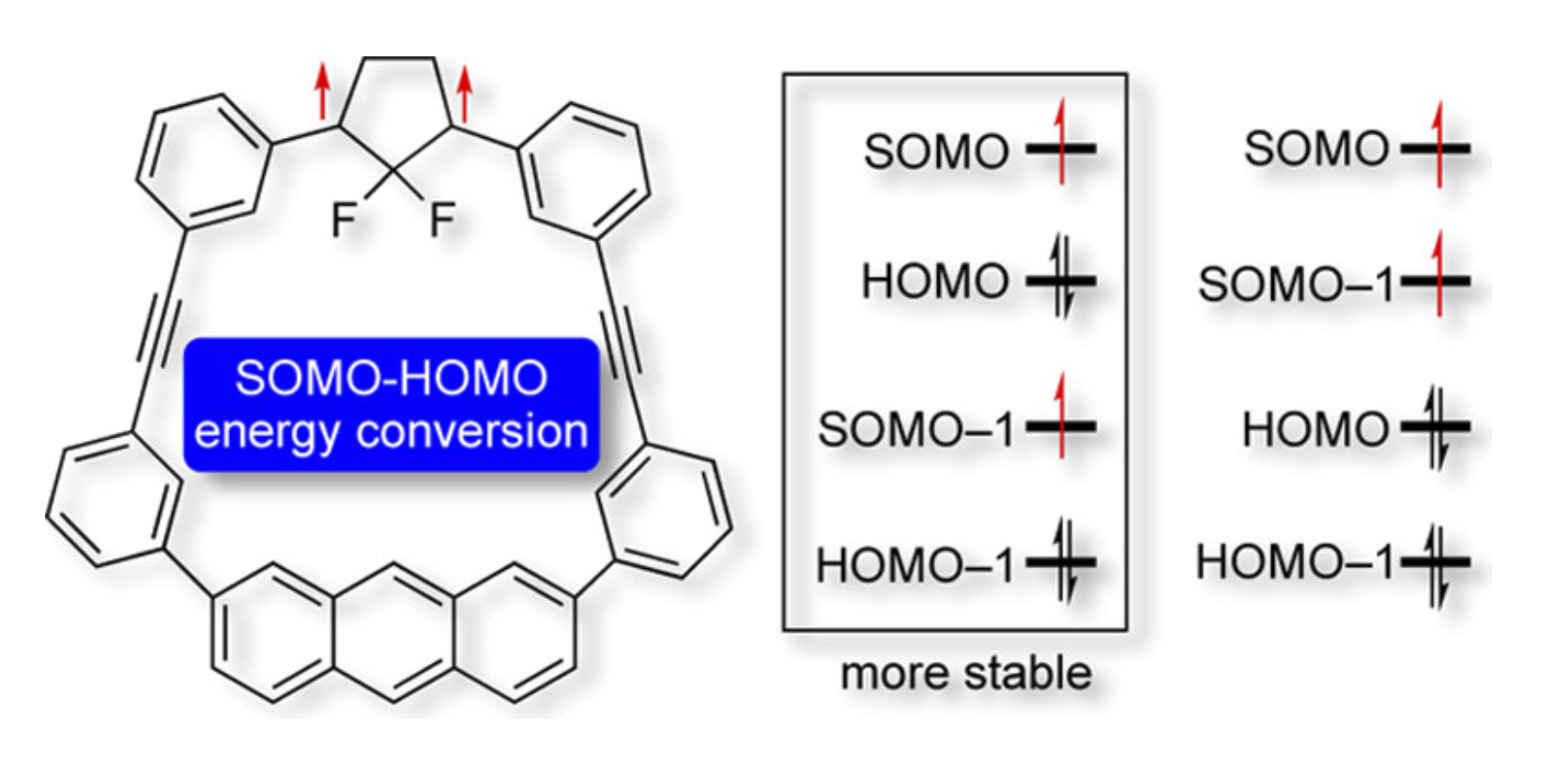
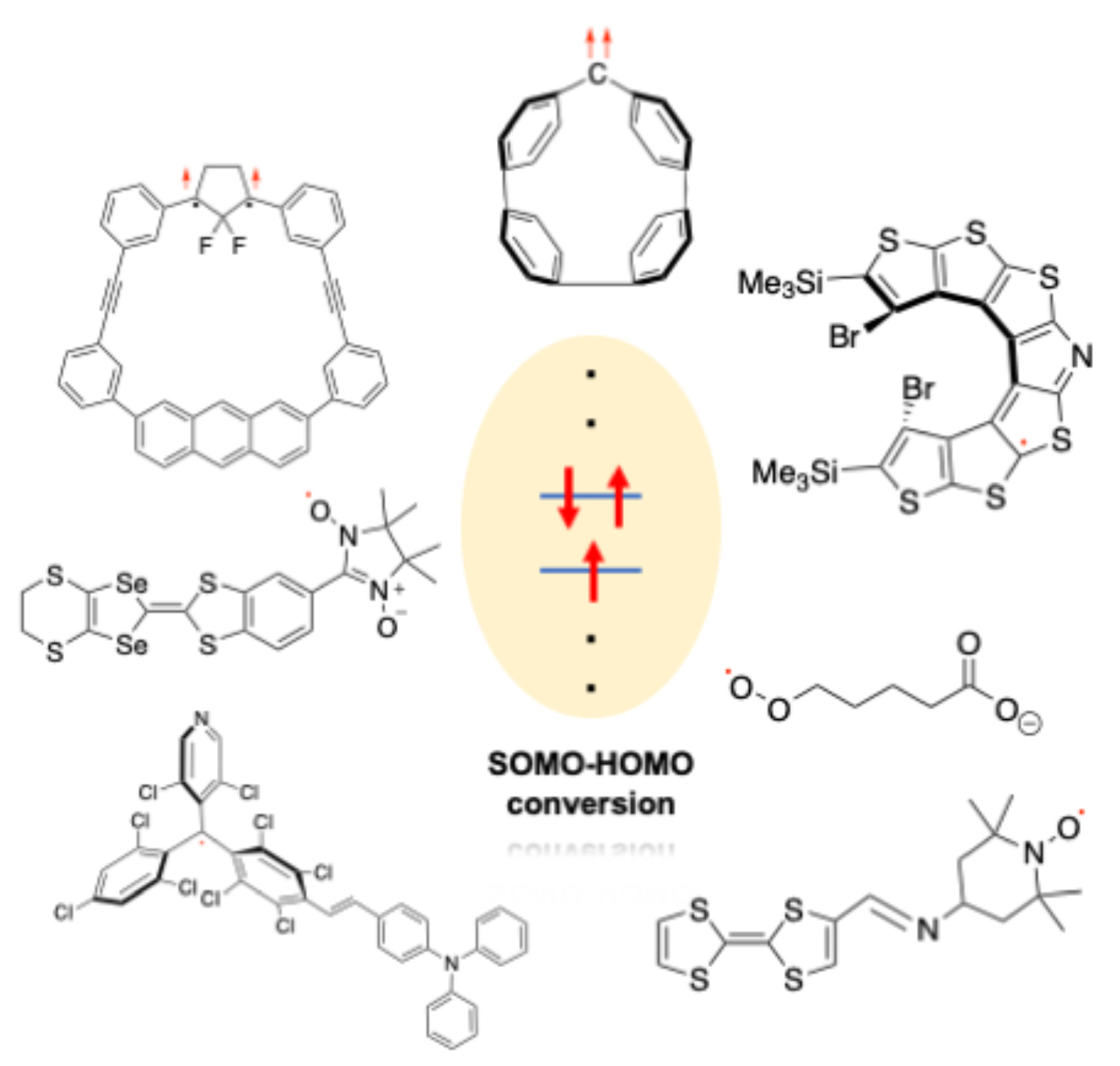 Singly Occupied Molecular Orbital−Highest Occupied Molecular Orbital (SOMO−HOMO) ConversionR. Murata, Z. Wang, and M. Abe*Aust. J. Chem., 2021, 74, 827–837.
Singly Occupied Molecular Orbital−Highest Occupied Molecular Orbital (SOMO−HOMO) ConversionR. Murata, Z. Wang, and M. Abe*Aust. J. Chem., 2021, 74, 827–837.Singly occupied molecular orbital−highest occupied molecular orbital (SOMO−HOMO) conversion (inversion), SHC, is a phenomenon in which the SOMO is lower in energy than the doubly occupied molecular orbitals (DOMO, HOMO). A non-Aufbau electronic structure leads to unique properties such as a switch in bond dissociation energy and the generation of high-spin species on one-electron oxidation. In addition, the pronounced photostability of these species has been reported recently for application in organic light-emitting devices. In this review article, we summarise the chemistry of SOMO−HOMO converted (inverted) species reported to date.
@article{murata2021singly, title = {Singly Occupied Molecular Orbital−Highest Occupied Molecular Orbital (SOMO−HOMO) Conversion}, author = {Murata, R. and Wang, Z. and Abe, M.}, journal = {Aust. J. Chem.}, volume = {74}, issue = {12}, pages = {827--837}, year = {2021}, month = oct, publisher = {csiro}, doi = {10.1071/ch21186}, url = {https://doi.org/10.1071/ch21186}, dimensions = {true}, tab = {review} } - Review
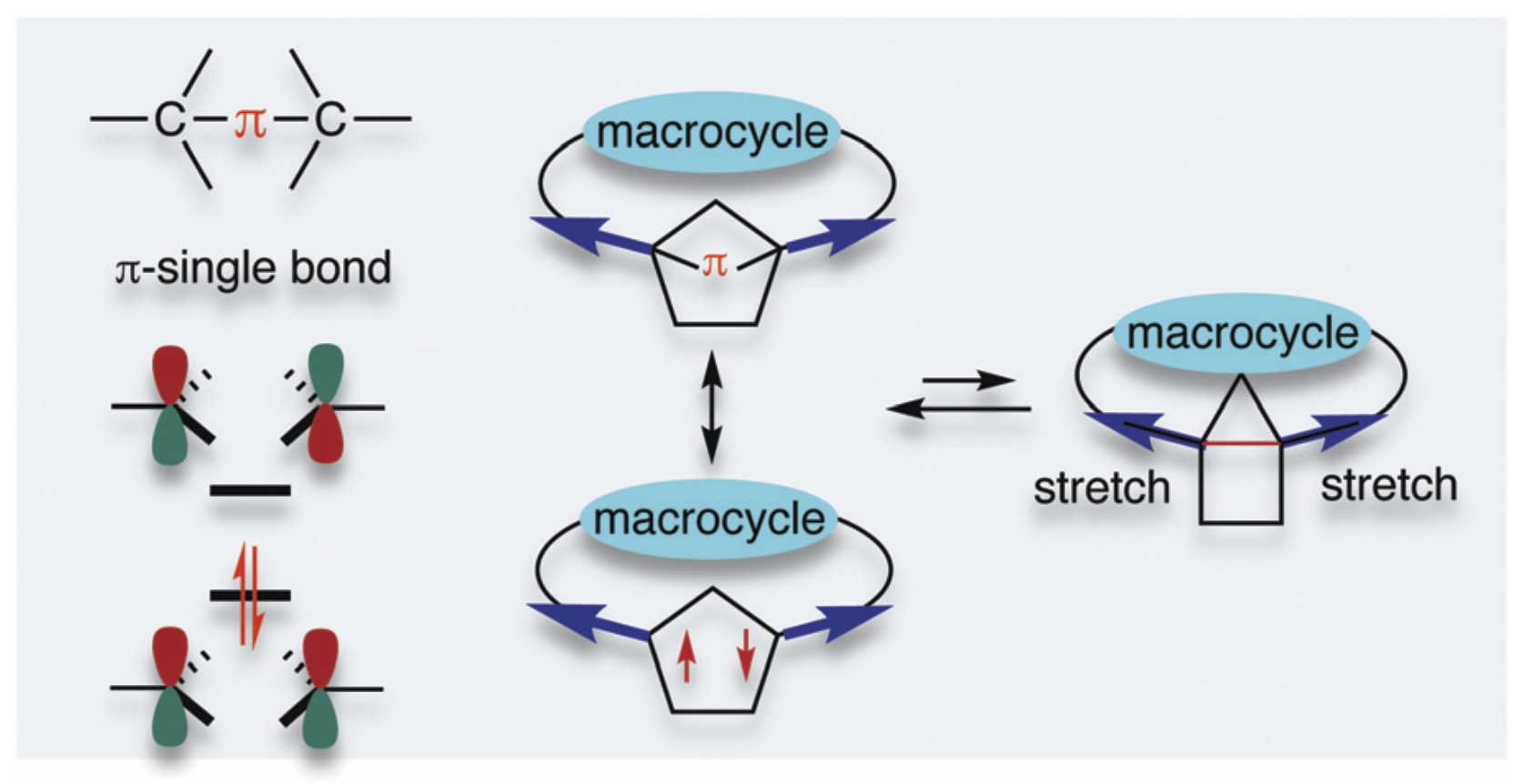 Long-lived localised singlet diradicaloids with carbon–carbon π-single bonding (C–π–C)Z. Wang, P. Yadav, and M. Abe*Chem. Commun., 2021, 57, 11301–11309.
Long-lived localised singlet diradicaloids with carbon–carbon π-single bonding (C–π–C)Z. Wang, P. Yadav, and M. Abe*Chem. Commun., 2021, 57, 11301–11309.Localised singlet cyclopentane-1,3-diyl diradicaloids have been considered promising candidates for constructing carbon–carbon π-single bonds (C–π–C). However, the high reactivity during formation of the σ-bond has limited a deeper investigation of its unique chemical properties. In this feature article, recent progress in kinetic stabilisation based on the “stretch effect” and the “solvent dynamic effect” induced by the macrocyclic system is summarised. Singlet diradicaloids S-DR4a/b and S-DR4d containing macrocyclic rings showed much longer lifetimes at 293 K (14 μs for S-DR4a and 156 μs for S-DR4b in benzene) compared to the parent singlet diradicaloid S-DR2 having no macrocyclic ring (209 ns in benzene). Furthermore, the dynamic solvent effect in viscous solvents was observed for the first time in intramolecular σ-bond formation, the lifetime of S-DR4d increased to 400 μs in the viscous solvent glycerin triacetin at 293 K. The experimental results proved the validity of the “stretch effect” and the “solvent dynamic effect” on the kinetic stabilisation of singlet cyclopentane-1,3-diyl diradicaloids, and provided a strategy for isolating the carbon–carbon π-single bonded species (C–π–C), and towards a deeper understanding of the nature of chemical bonding.
@article{wang2021long, title = {Long-lived localised singlet diradicaloids with carbon–carbon π-single bonding (C–π–C)}, author = {Wang, Z. and Yadav, P. and Abe, M.}, journal = {Chem. Commun.}, volume = {57}, issue = {86}, pages = {11301--11309}, year = {2021}, month = sep, publisher = {rsc}, doi = {10.1039/d1cc04581d}, url = {https://doi.org/10.1039/d1cc04581d}, dimensions = {true}, tab = {review} } -
 SOMO–HOMO Conversion in Triplet Cyclopentane-1,3-diyl DiradicalsZ. Wang, R. Murata, and M. Abe*ACS Omega, 2021, 6, 22773–22779.
SOMO–HOMO Conversion in Triplet Cyclopentane-1,3-diyl DiradicalsZ. Wang, R. Murata, and M. Abe*ACS Omega, 2021, 6, 22773–22779.According to the Aufbau principle, singly occupied molecular orbitals (SOMOs) are energetically higher lying than a highest doubly occupied molecular orbital (HOMO) in the electronically ground state of radicals. However, in the last decade, SOMO–HOMO-converted species have been reported in a limited group of radicals, such as distonic anion radicals and nitroxides. In this study, SOMO–HOMO conversion was observed in triplet 2,2-difluorocyclopentane-1,3-diyl diradicals DR3F1, DR4F1, and 2-fluorocyclopentante-1,3-diyl diradical DR3HF1, which contain the anthracyl unit at the remote position. The high HOMO energy in the anthracyl moiety and the low-lying SOMO–1 due to the fluoro-substituent effect are the key to the SOMO–HOMO conversion phenomenon. Furthermore, the cation radical DR3F1+ generated through the one-electron oxidation of DR3F1 was found to be a SOMO–HOMO-converted monoradical.
@article{wang2021somo, title = {SOMO–HOMO Conversion in Triplet Cyclopentane-1,3-diyl Diradicals}, author = {Wang, Z. and Murata, R. and Abe, M.}, journal = {ACS Omega}, volume = {6}, issue = {35}, pages = {22773--22779}, year = {2021}, month = jul, publisher = {acs}, doi = {10.1021/acsomega.1c03125}, url = {https://doi.org/10.1021/acsomega.1c03125}, dimensions = {true}, tab = {paper} } - Cover Picture
 SOMO–HOMO Conversion in Triplet CarbenesOrg. Lett., 2021, 23, 4955–4959.
SOMO–HOMO Conversion in Triplet CarbenesOrg. Lett., 2021, 23, 4955–4959.In this study, the SOMO–HOMO conversion has been shown for the first time in triplet carbenes embedded in cycloparaphenylene units. The high-lying HOMO originating from the curved π-conjugated system and the low-lying SOMO–1 originating due to the small carbene angle are the key to endowing this interesting electronic configuration. Furthermore, simple planar triplet carbenes such as fluorenylidene were found to possess SOMO–HOMO energy-converted electronic configurations.
@article{murata2021somo, title = {SOMO–HOMO Conversion in Triplet Carbenes}, author = {Murata, R. and Wang, Z. and Miyazawa, Y. and Antol, I. and Yamago, S. and Abe, M.}, journal = {Org. Lett.}, volume = {23}, issue = {13}, pages = {4955--4959}, year = {2021}, month = may, publisher = {acs}, doi = {10.1021/acs.orglett.1c01137}, url = {https://doi.org/10.1021/acs.orglett.1c01137}, dimensions = {true}, tab = {paper} } - Cover Picture
 1,3-Diradicals Embedded in Curved Paraphenylene Units: Singlet versus Triplet State and In-Plane AromaticityJ. Am. Chem. Soc., 2021, 143, 7426–7439.
1,3-Diradicals Embedded in Curved Paraphenylene Units: Singlet versus Triplet State and In-Plane AromaticityJ. Am. Chem. Soc., 2021, 143, 7426–7439.Curved π-conjugated molecules and open-shell structures have attracted much attention from the perspective of fundamental chemistry, as well as materials science. In this study, the chemistry of 1,3-diradicals (DRs) embedded in curved cycloparaphenylene (CPPs) structures, DR-(n+3)CPPs (n = 0–5), was investigated to understand the effects of the curvature and system size on the spin–spin interactions and singlet versus triplet state, as well as their unique characteristics such as in-plane aromaticity. A triplet ground state was predicted for the larger 1,3-diradicals, such as the seven- and eight-paraphenylene-unit-containing diradicals DR-7CPP (n = 4) and DR-8CPP (n = 5), by quantum chemical calculations. The smaller-sized diradicals DR-(n+3)CPPs (n = 0–3) were found to possess singlet ground states. Thus, the ground-state spin multiplicity is controlled by the size of the paraphenylene cycle. The size effect on the ground-state spin multiplicity was confirmed by the experimental generation of DR-6CPP in the photochemical denitrogenation of its azo-containing precursor (AZ-6CPP). Intriguingly, a unique type of in-plane aromaticity emerged in the smaller-sized singlet states such as S-DR-4CPP (n = 1), as proven by nucleus-independent chemical shift calculations (NICS) and an analysis of the anisotropy of the induced current density (ACID), which demonstrate that homoconjugation between the 1,3-diradical moiety arises because of the curved and distorted bonding system.
@article{miyazawa2021diradicals, title = {1,3-Diradicals Embedded in Curved Paraphenylene Units: Singlet versus Triplet State and In-Plane Aromaticity}, author = {Miyazawa, Y. and Wang, Z. and Matsumoto, M. and Hatano, S. and Antol, I. and Kayahara, E. and Yamago, S. and Abe, M.}, journal = {J. Am. Chem. Soc.}, volume = {143}, issue = {19}, pages = {7426--7439}, year = {2021}, month = apr, publisher = {acs}, doi = {10.1021/jacs.1c01329}, url = {https://doi.org/10.1021/jacs.1c01329}, dimensions = {true}, tab = {paper} } - HOT Article
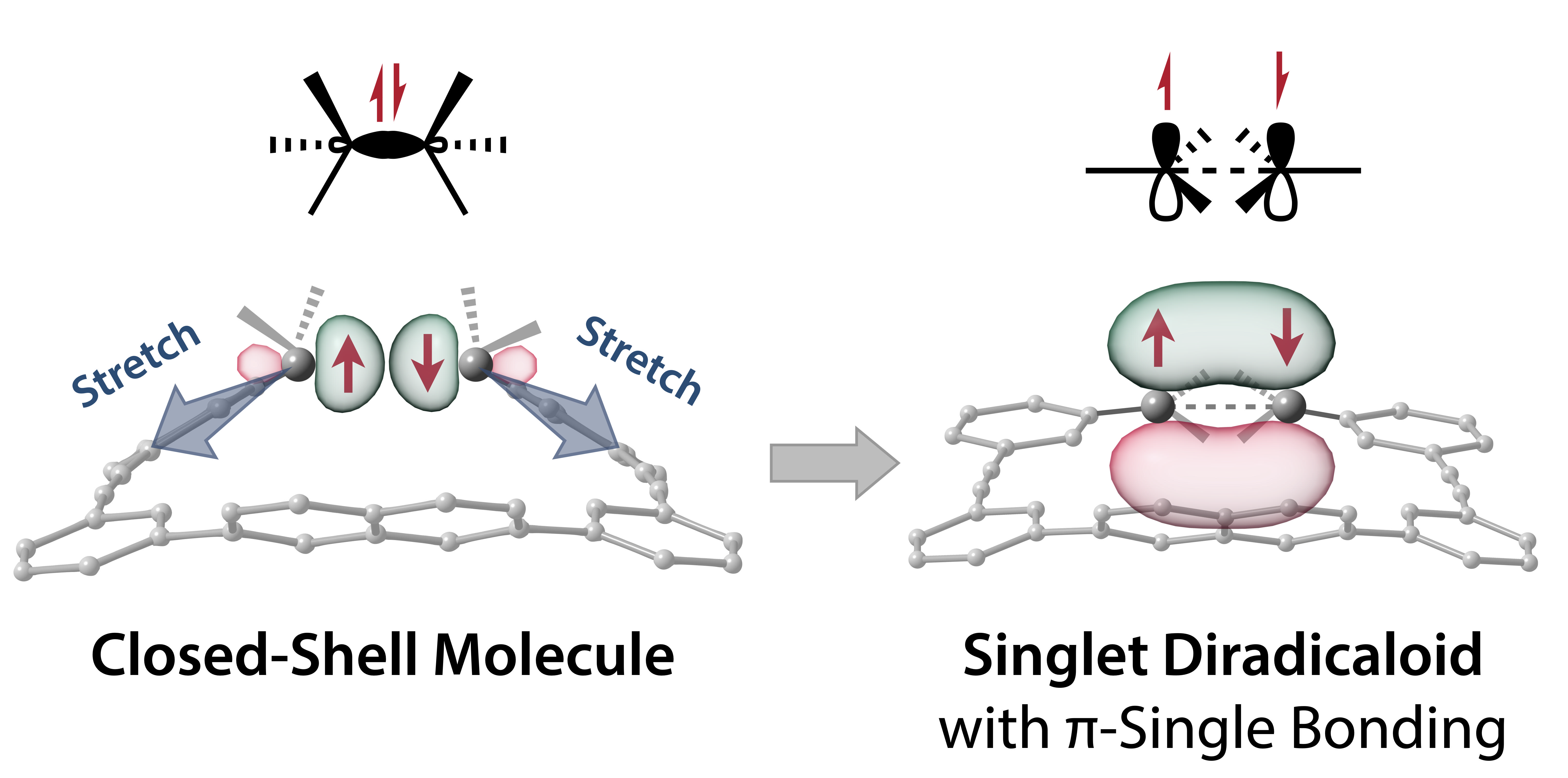 Impact of the macrocyclic structure and dynamic solvent effect on the reactivity of a localised singlet diradicaloid with π-single bonding characterZ. Wang, R. Akisaka, S. Yabumoto, T. Nakagawa, S. Hatano, and M. Abe*Chem. Sci., 2021, 12, 613–625.
Impact of the macrocyclic structure and dynamic solvent effect on the reactivity of a localised singlet diradicaloid with π-single bonding characterZ. Wang, R. Akisaka, S. Yabumoto, T. Nakagawa, S. Hatano, and M. Abe*Chem. Sci., 2021, 12, 613–625.Localised singlet diradicals are key intermediates in bond homolysis processes. Generally, these highly reactive species undergo radical–radical coupling reaction immediately after their generation. Therefore, their short-lived character hampers experimental investigations of their nature. In this study, we implemented the new concept of “stretch effect” to access a kinetically stabilised singlet diradicaloid. To this end, a macrocyclic structure was computationally designed to enable the experimental examination of a singlet diradicaloid with π-single bonding character. The kinetically stabilised diradicaloid exhibited a low carbon–carbon coupling reaction rate of 6.4 × 103 s−1 (155.9 μs), approximately 11 and 1000 times slower than those of the first generation of macrocyclic system (7.0 × 104 s−1, 14.2 μs) and the parent system lacking the macrocycle (5 × 106 s−1, 200 ns) at 293 K in benzene, respectively. In addition, a significant dynamic solvent effect was observed for the first time in intramolecular radical–radical coupling reactions in viscous solvents such as glycerin triacetate. This theoretical and experimental study demonstrates that the stretch effect and solvent viscosity play important roles in retarding the σ-bond formation process, thus enabling a thorough examination of the nature of the singlet diradicaloid and paving the way toward a deeper understanding of reactive intermediates.
@article{wang2021stretch, title = {Impact of the macrocyclic structure and dynamic solvent effect on the reactivity of a localised singlet diradicaloid with π-single bonding character}, author = {Wang, Z. and Akisaka, R. and Yabumoto, S. and Nakagawa, T. and Hatano, S. and Abe, M.}, journal = {Chem. Sci.}, volume = {12}, issue = {2}, pages = {613--625}, year = {2021}, month = jan, publisher = {rsc}, doi = {10.1039/d0sc05311b}, url = {https://doi.org/10.1039/d0sc05311b}, dimensions = {true}, tab = {paper} }