publications
公表論文
List by Type: original papers / accounts & reviews / cover pictures
List by Year: 2025 / 2024 / 2023 / 2022 / 2021 / 2020 / 2019 / 2018
2025
-
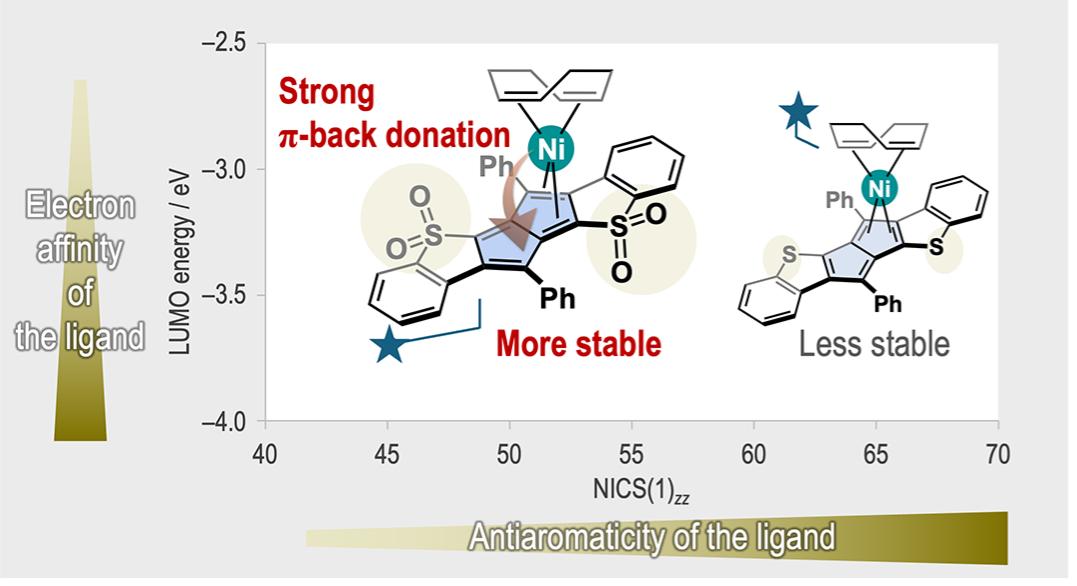 A Persistent Ni(0)–Pentalene Complex with High Fluxionality: Does Stronger Antiaromaticity Promote Metal–Ligand Interactions?R. Kuwata, T. Imanishi, S. Hasegawa, Z. Wang, J. Usuba, K. Yasui, M. U. G. Khan, J. I. Wu*, Y. Uetake*, and A. Fukazawa*ChemRxiv, 2025, Preprint.
A Persistent Ni(0)–Pentalene Complex with High Fluxionality: Does Stronger Antiaromaticity Promote Metal–Ligand Interactions?R. Kuwata, T. Imanishi, S. Hasegawa, Z. Wang, J. Usuba, K. Yasui, M. U. G. Khan, J. I. Wu*, Y. Uetake*, and A. Fukazawa*ChemRxiv, 2025, Preprint.Despite the long-standing use of transition metal complexation for stabilizing antiaromatic hydrocarbons, the underlying factors that govern their coordination behavior remain unclear. A Ni(0) complex, [Ni(cod)(1)], featuring a benzothiophene-S,S-dioxide-fused pentalene ligand, was synthesized and characterized, and compared to a benzothiophene-fused analog, [Ni(cod)(2)], which bears a more antiaromatic yet less electron-accepting pentalene ligand. Single-crystal X-ray diffraction, X-ray absorption spectroscopy, and variable-temperature NMR, combined with computational analyses, reveal that the enhanced stability of [Ni(cod)(1)] arises primarily from substantial π-back donation facilitated by strongly electron-withdrawing SO2 groups that lower the LUMO energy of 1. Comparisons to [Ni(cod)(2)] further demonstrate that a more antiaromatic core does not necessarily lead to stronger coordination to the Ni center. Surprisingly, the robust [Ni(cod)(1)] complex is fluxional in solution; the Ni center migrates reversibly between the two five-membered rings of the pentalene ligand via a bond shift mechanism.
@article{kuwata2025a, title = {A Persistent Ni(0)–Pentalene Complex with High Fluxionality: Does Stronger Antiaromaticity Promote Metal–Ligand Interactions?}, author = {Kuwata, R. and Imanishi, T. and Hasegawa, S. and Wang, Z. and Usuba, J. and Yasui, K. and Khan, M. U. G. and Wu, J. I. and Uetake, Y. and Fukazawa, A.}, journal = {ChemRxiv}, year = {2025}, pages = {Preprint}, month = nov, doi = {10.26434/chemrxiv-2025-l8c87-v2}, url = {https://doi.org/10.26434/chemrxiv-2025-l8c87-v2}, dimensions = {true}, tab = {paper} }
2024
-
 py.Aroma: An Intuitive Graphical User Interface for Diverse Aromaticity AnalysesZ. Wang*Chemistry, 2024, 6, 1692–1703.
py.Aroma: An Intuitive Graphical User Interface for Diverse Aromaticity AnalysesZ. Wang*Chemistry, 2024, 6, 1692–1703.The nucleus-independent chemical shift (NICS) criterion plays a significant role in evaluating (anti-)aromaticity. While readily accessible even for non-computational chemists, adding ghost atoms for multi-points NICS evaluations poses a significant challenge. In this article, I introduce py.Aroma, a freely available and open-source Python package designed specifically for analyzing (anti-)aromaticity. Through its user-friendly graphical interface, py.Aroma simplifies and enhances aromaticity analyses by offering key features such as HOMA/HOMER index computation, Gaussian-type input file generation for diverse NICS calculations and corresponding output processing, NMR spectra plotting, and generating computational supporting information (SI) for scientific manuscripts. Additionally, NICS⊥ is suggested for evaluating (anti-)aromaticity for non-planar or tilted rings. Pre-compiled executables for macOS and Windows are available at https://wongzit.github.io/program/pyaroma. Make facilitate accessibility for users lacking programming experience or time constraints.
@article{wang2024pyaroma, title = {py.Aroma: An Intuitive Graphical User Interface for Diverse Aromaticity Analyses}, author = {Wang, Z.}, journal = {Chemistry}, volume = {6}, issue = {6}, pages = {1692--1703}, year = {2024}, month = dec, publisher = {MDPI}, doi = {10.3390/chemistry6060103}, url = {https://doi.org/10.3390/chemistry6060103}, dimensions = {true}, tab = {paper} }
2023
- Cover Picture
 Energetically More Stable Singlet Cyclopentane-1,3-diyl Diradical with π-Single Bonding Character than the Corresponding σ-Single Bonded CompoundQ. Liu, K. Onishi, Y. Miyazawa, Z. Wang, S. Hatano, and M. Abe*J. Am. Chem. Soc., 2023, 145, 27089–27094.
Energetically More Stable Singlet Cyclopentane-1,3-diyl Diradical with π-Single Bonding Character than the Corresponding σ-Single Bonded CompoundQ. Liu, K. Onishi, Y. Miyazawa, Z. Wang, S. Hatano, and M. Abe*J. Am. Chem. Soc., 2023, 145, 27089–27094.Carbon–carbon σ-single bonds are crucial for constructing molecules like ethane derivatives (R3C–CR3), which are composed of tetrahedral four-coordinate carbons. Molecular functions, such as light absorption or emission, originate from the π-bonds existing in ethylene derivatives (R2C═CR2). In this study, a relatively stable cyclopentane-1,3-diyl species with π-single bonding system (C−π–C) with planar four-coordinate carbons is constructed. This diradicaloid is energetically more stable than the corresponding σ-single bonding system. The π-electron single bonding system provides deeper insights into the chemical bonding and the physical properties derived from the small energy gaps between the bonding and antibonding molecular orbitals.
@article{onishi2023energetically, title = {Energetically More Stable Singlet Cyclopentane-1,3-diyl Diradical with π-Single Bonding Character than the Corresponding σ-Single Bonded Compound}, author = {Liu, Q. and Onishi, K. and Miyazawa, Y. and Wang, Z. and Hatano, S. and Abe, M.}, journal = {J. Am. Chem. Soc.}, volume = {145}, issue = {49}, pages = {27089--27094}, year = {2023}, month = nov, publisher = {acs}, doi = {10.1021/jacs.3c10971}, url = {https://doi.org/10.1021/jacs.3c10971}, dimensions = {true}, tab = {paper} } -
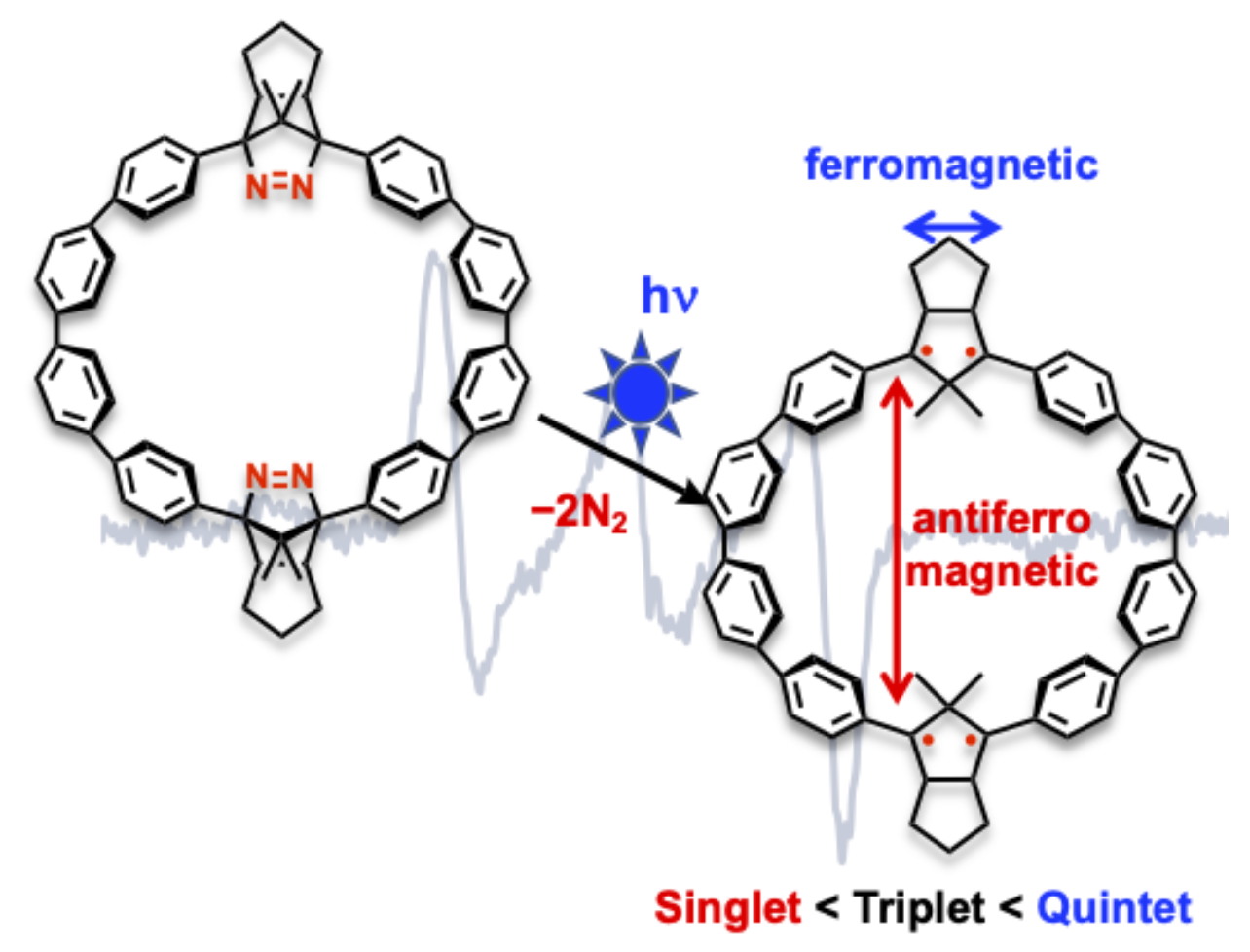 Generation and Characterization of a Tetraradical Embedded in a Curved Cyclic Paraphenylene UnitY. Miyazawa, Z. Wang, S. Hatano, R. Takagi, H. Matsuoka*, N. Amamizu, Y. Kitagawa*, E. Kayahara, S. Yamago, and M. Abe*Chem. Eur. J., 2023, 29, e202301009.
Generation and Characterization of a Tetraradical Embedded in a Curved Cyclic Paraphenylene UnitY. Miyazawa, Z. Wang, S. Hatano, R. Takagi, H. Matsuoka*, N. Amamizu, Y. Kitagawa*, E. Kayahara, S. Yamago, and M. Abe*Chem. Eur. J., 2023, 29, e202301009.Unique spin–spin (magnetic) interactions, ring-size effects on ground-state spin multiplicity, and in-plane aromaticity has been found in localized 1,3-diradicals embedded in curved benzene structures such as cycloparaphenylene (CPP). In this study, we characterized the magnetic interactions in a tetraradical consisting of two localized 1,3-diradical units connected by p-quaterphenyl within a curved CPP skeleton by electron paramagnetic resonance (EPR) spectroscopy and quantum chemical calculations. Persistent triplet species with zero-field splitting parameters similar to those of a triplet 1,3-diphenylcyclopentane-1,3-diyl diradical were observed by continuous wave (CW) or pulsed X-band EPR measurements. The quintet state derived from the ferromagnetic interaction between the two triplet diradical moieties was not detected at 20 K under glassy matrix conditions. At the B3LYP/6-31G(d) level of theory, the singlet state was lower in energy than the triplet and quintet states. These findings will aid in the development of open-shell species for material science application.
@article{miyazawa2023generation, title = {Generation and Characterization of a Tetraradical Embedded in a Curved Cyclic Paraphenylene Unit}, author = {Miyazawa, Y. and Wang, Z. and Hatano, S. and Takagi, R. and Matsuoka, H. and Amamizu, N. and Kitagawa, Y. and Kayahara, E. and Yamago, S. and Abe, M.}, journal = {Chem. Eur. J.}, volume = {29}, issue = {42}, pages = {e202301009}, year = {2023}, month = may, publisher = {wiley}, doi = {10.1002/chem.202301009}, url = {https://doi.org/10.1002/chem.202301009}, dimensions = {true}, tab = {paper} }
2022
-
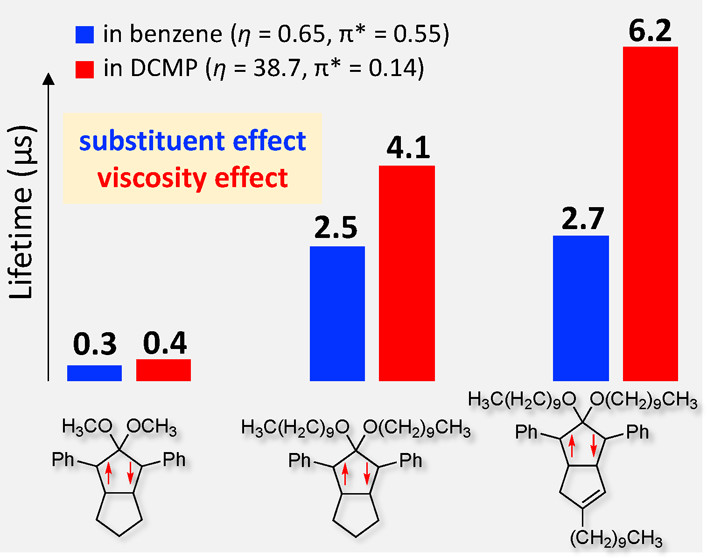 Impacts of Solvent and Alkyl Chain Length on the Lifetime of Singlet Cyclopentane-1,3-diyl Diradicaloids with π-Single BondingQ. Liu, Z. Wang, and M. Abe*J. Org. Chem., 2022, 87, 1858–1866.
Impacts of Solvent and Alkyl Chain Length on the Lifetime of Singlet Cyclopentane-1,3-diyl Diradicaloids with π-Single BondingQ. Liu, Z. Wang, and M. Abe*J. Org. Chem., 2022, 87, 1858–1866.The singlet 2,2-dialkoxycyclopentane-1,3-diyl diradicaloids are not only the important key intermediates in the process of bond homolysis but are also attracting attention as π-single bonding compounds. In the present study, the effects of solvent viscosity η (0.24–125.4 mPa s) and polarity π* (−0.11 to 1.00 kcal mol–1) on the reactivity of localized singlet diradicaloids were thoroughly investigated using 18 different solvents including binary mixed solvent systems containing ionic liquids. In low-η solvents (η < 1 mPa s), the lifetimes of singlet diradicaloids, which are determined by the rate constant for the isomerization of π-single-bonded singlet diradicaloids to the σ-bonded isomer, were substantially dependent on π*. Slower isomerization was observed in more polar solvents. In high-η solvents (η > 2 mPa s), the rate of isomerization was largely influenced by η in addition to π*. Slower isomerization was observed in more viscous solvents. Experimental results demonstrated the crucial roles of both solvent polarity and viscosity in the reactivity of singlet diradicaloids and thus clarified the characters of singlet diradicaloids and molecular motions during the chemical transformation. The dynamic solvent effect was further proved by a long alkyl chain introduced at a remote position of the reaction site.
@article{liu2022impacts, title = {Impacts of Solvent and Alkyl Chain Length on the Lifetime of Singlet Cyclopentane-1,3-diyl Diradicaloids with π-Single Bonding}, author = {Liu, Q. and Wang, Z. and Abe, M.}, journal = {J. Org. Chem.}, volume = {87}, issue = {3}, pages = {1858--1866}, year = {2022}, month = jan, publisher = {acs}, doi = {10.1021/acs.joc.1c02895}, url = {https://doi.org/10.1021/acs.joc.1c02895}, dimensions = {true}, tab = {paper} }
2021
- Review
 New Insights into Bond Homolysis Process and Discovery of Novel Bonding System (C–π–C) by Generating Long-lived Singlet DiradicalsM. Abe*, Z. Wang, and R. AkisakaAsiaChem, 2021, 2, 32–41.
New Insights into Bond Homolysis Process and Discovery of Novel Bonding System (C–π–C) by Generating Long-lived Singlet DiradicalsM. Abe*, Z. Wang, and R. AkisakaAsiaChem, 2021, 2, 32–41.In recent years, low-valent chemical species such as radicals and carbenes, which have been recognized as short-lived intermediates, have been isolated by appropriate molecular design, and their chemical properties have been investigated in detail experimentally. In particular, the discovery of isolable carbenes, which are now widely used as indispensable ligands in coordination chemistry and synthetic organic chemistry, has enabled the development of novel highly active catalysts.
@article{abe2021new, title = {New Insights into Bond Homolysis Process and Discovery of Novel Bonding System (C–π–C) by Generating Long-lived Singlet Diradicals}, author = {Abe, M. and Wang, Z. and Akisaka, R.}, journal = {AsiaChem}, volume = {2}, issue = {1}, pages = {32--41}, year = {2021}, month = dec, publisher = {ics}, doi = {10.51167/acm00020.51167/acm00021}, url = {https://doi.org/10.51167/acm00020.51167/acm00021}, dimensions = {true}, tab = {review} } - Review
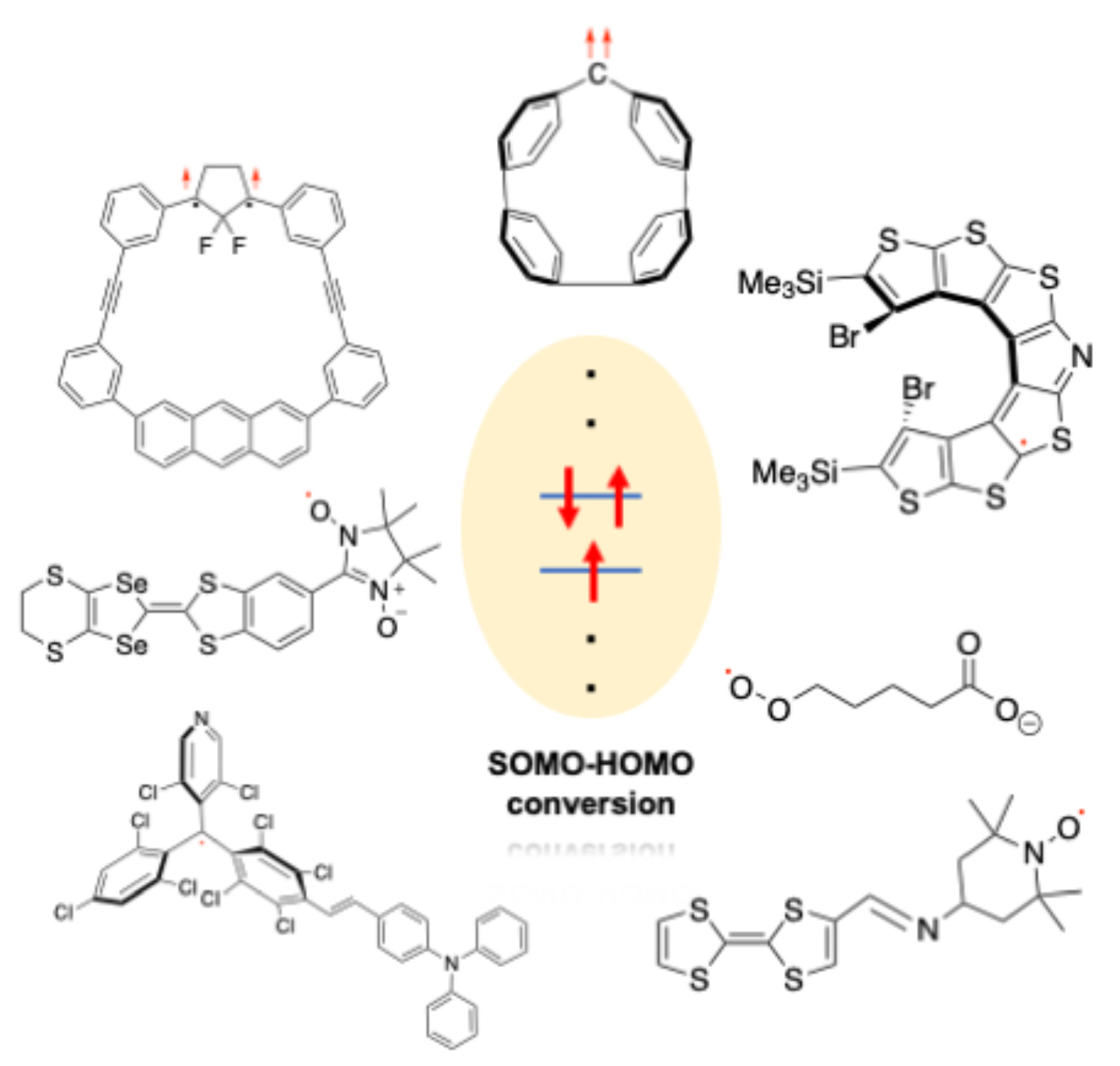 Singly Occupied Molecular Orbital−Highest Occupied Molecular Orbital (SOMO−HOMO) ConversionR. Murata, Z. Wang, and M. Abe*Aust. J. Chem., 2021, 74, 827–837.
Singly Occupied Molecular Orbital−Highest Occupied Molecular Orbital (SOMO−HOMO) ConversionR. Murata, Z. Wang, and M. Abe*Aust. J. Chem., 2021, 74, 827–837.Singly occupied molecular orbital−highest occupied molecular orbital (SOMO−HOMO) conversion (inversion), SHC, is a phenomenon in which the SOMO is lower in energy than the doubly occupied molecular orbitals (DOMO, HOMO). A non-Aufbau electronic structure leads to unique properties such as a switch in bond dissociation energy and the generation of high-spin species on one-electron oxidation. In addition, the pronounced photostability of these species has been reported recently for application in organic light-emitting devices. In this review article, we summarise the chemistry of SOMO−HOMO converted (inverted) species reported to date.
@article{murata2021singly, title = {Singly Occupied Molecular Orbital−Highest Occupied Molecular Orbital (SOMO−HOMO) Conversion}, author = {Murata, R. and Wang, Z. and Abe, M.}, journal = {Aust. J. Chem.}, volume = {74}, issue = {12}, pages = {827--837}, year = {2021}, month = oct, publisher = {csiro}, doi = {10.1071/ch21186}, url = {https://doi.org/10.1071/ch21186}, dimensions = {true}, tab = {review} } - Review
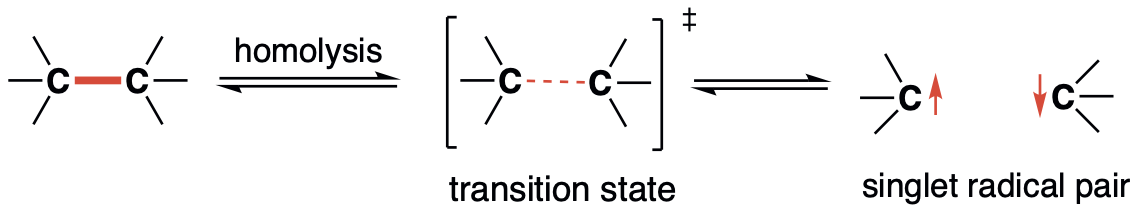
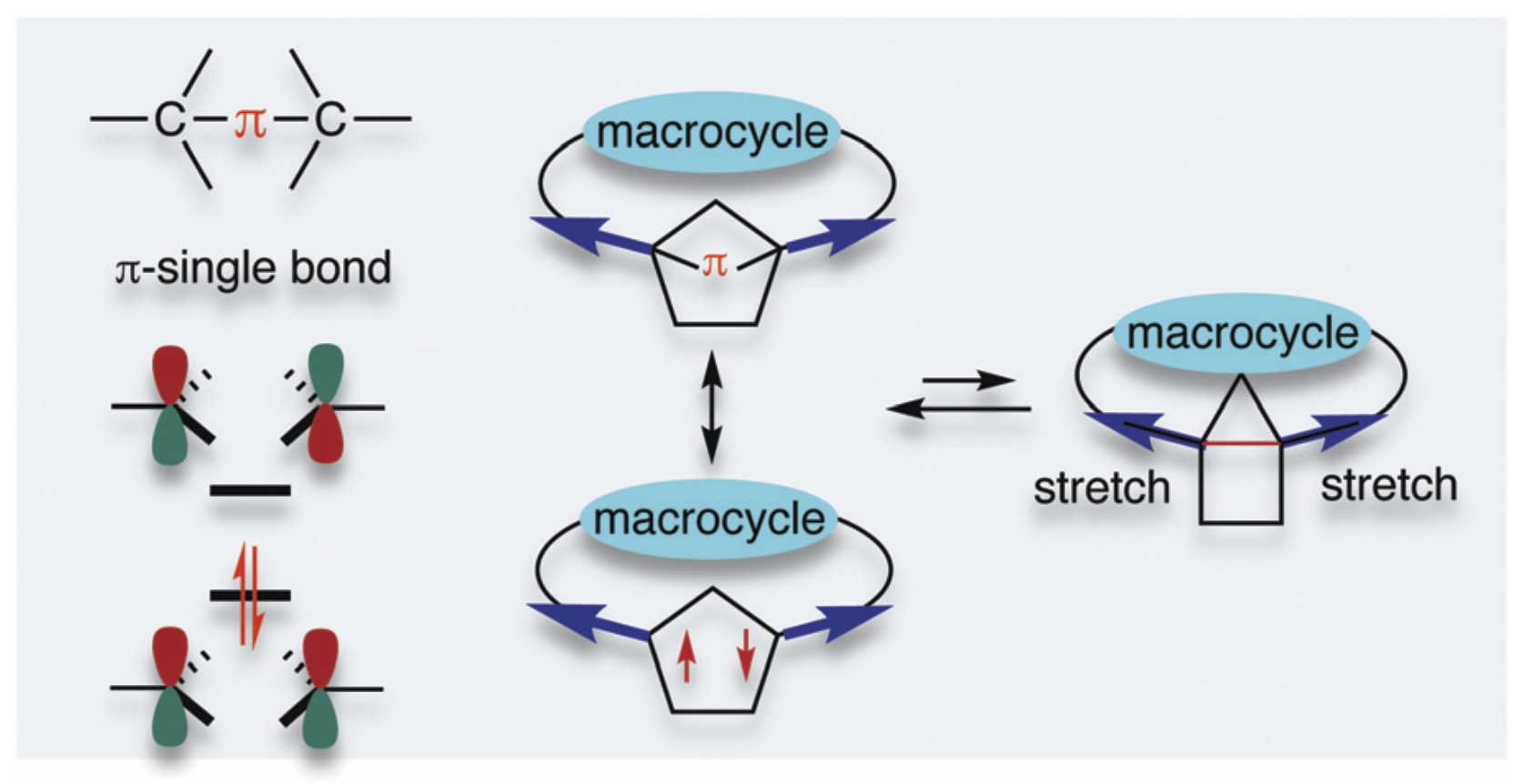 Long-lived localised singlet diradicaloids with carbon–carbon π-single bonding (C–π–C)Z. Wang, P. Yadav, and M. Abe*Chem. Commun., 2021, 57, 11301–11309.
Long-lived localised singlet diradicaloids with carbon–carbon π-single bonding (C–π–C)Z. Wang, P. Yadav, and M. Abe*Chem. Commun., 2021, 57, 11301–11309.Localised singlet cyclopentane-1,3-diyl diradicaloids have been considered promising candidates for constructing carbon–carbon π-single bonds (C–π–C). However, the high reactivity during formation of the σ-bond has limited a deeper investigation of its unique chemical properties. In this feature article, recent progress in kinetic stabilisation based on the “stretch effect” and the “solvent dynamic effect” induced by the macrocyclic system is summarised. Singlet diradicaloids S-DR4a/b and S-DR4d containing macrocyclic rings showed much longer lifetimes at 293 K (14 μs for S-DR4a and 156 μs for S-DR4b in benzene) compared to the parent singlet diradicaloid S-DR2 having no macrocyclic ring (209 ns in benzene). Furthermore, the dynamic solvent effect in viscous solvents was observed for the first time in intramolecular σ-bond formation, the lifetime of S-DR4d increased to 400 μs in the viscous solvent glycerin triacetin at 293 K. The experimental results proved the validity of the “stretch effect” and the “solvent dynamic effect” on the kinetic stabilisation of singlet cyclopentane-1,3-diyl diradicaloids, and provided a strategy for isolating the carbon–carbon π-single bonded species (C–π–C), and towards a deeper understanding of the nature of chemical bonding.
@article{wang2021long, title = {Long-lived localised singlet diradicaloids with carbon–carbon π-single bonding (C–π–C)}, author = {Wang, Z. and Yadav, P. and Abe, M.}, journal = {Chem. Commun.}, volume = {57}, issue = {86}, pages = {11301--11309}, year = {2021}, month = sep, publisher = {rsc}, doi = {10.1039/d1cc04581d}, url = {https://doi.org/10.1039/d1cc04581d}, dimensions = {true}, tab = {review} } -
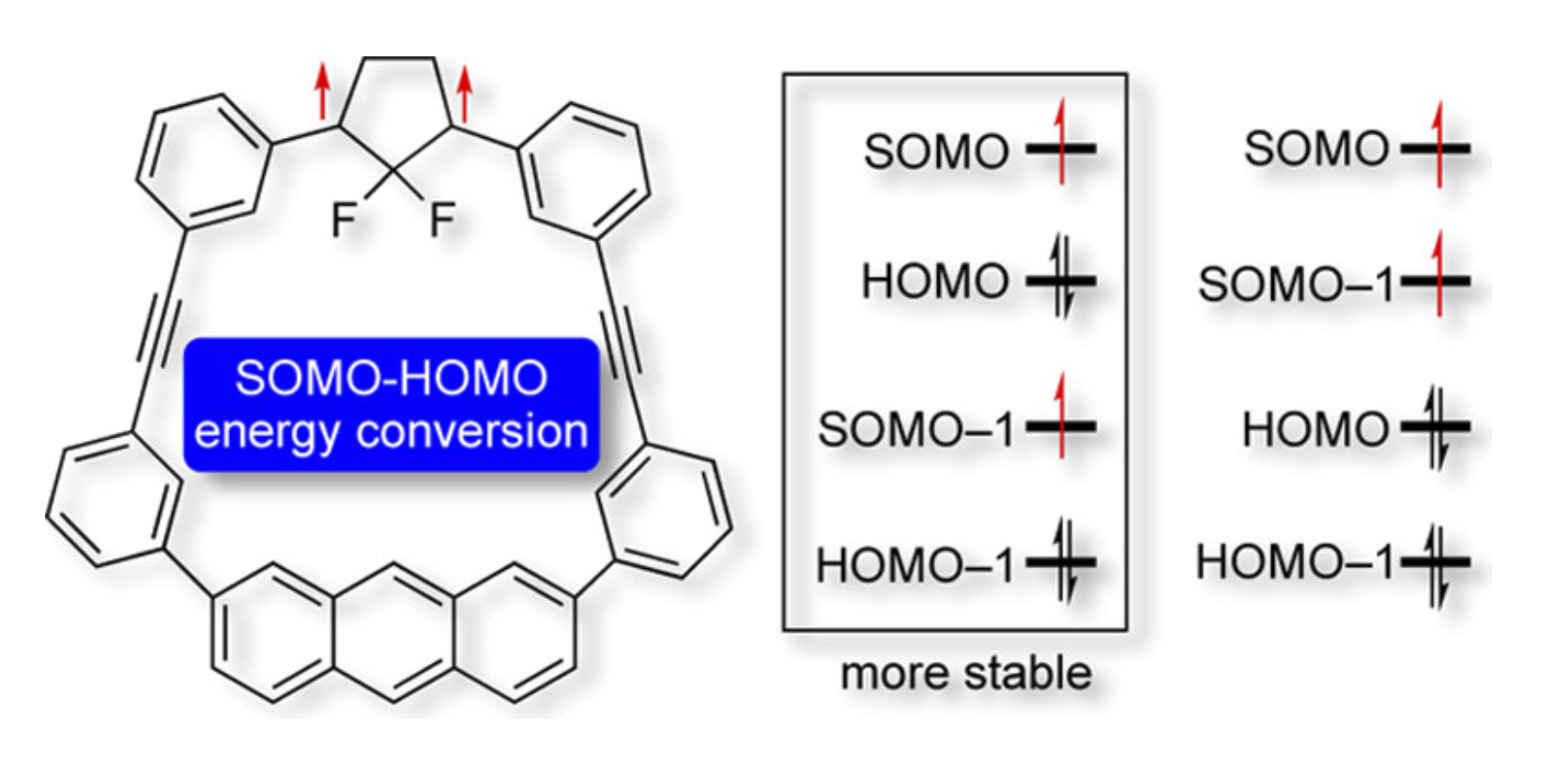 SOMO–HOMO Conversion in Triplet Cyclopentane-1,3-diyl DiradicalsZ. Wang, R. Murata, and M. Abe*ACS Omega, 2021, 6, 22773–22779.
SOMO–HOMO Conversion in Triplet Cyclopentane-1,3-diyl DiradicalsZ. Wang, R. Murata, and M. Abe*ACS Omega, 2021, 6, 22773–22779.According to the Aufbau principle, singly occupied molecular orbitals (SOMOs) are energetically higher lying than a highest doubly occupied molecular orbital (HOMO) in the electronically ground state of radicals. However, in the last decade, SOMO–HOMO-converted species have been reported in a limited group of radicals, such as distonic anion radicals and nitroxides. In this study, SOMO–HOMO conversion was observed in triplet 2,2-difluorocyclopentane-1,3-diyl diradicals DR3F1, DR4F1, and 2-fluorocyclopentante-1,3-diyl diradical DR3HF1, which contain the anthracyl unit at the remote position. The high HOMO energy in the anthracyl moiety and the low-lying SOMO–1 due to the fluoro-substituent effect are the key to the SOMO–HOMO conversion phenomenon. Furthermore, the cation radical DR3F1+ generated through the one-electron oxidation of DR3F1 was found to be a SOMO–HOMO-converted monoradical.
@article{wang2021somo, title = {SOMO–HOMO Conversion in Triplet Cyclopentane-1,3-diyl Diradicals}, author = {Wang, Z. and Murata, R. and Abe, M.}, journal = {ACS Omega}, volume = {6}, issue = {35}, pages = {22773--22779}, year = {2021}, month = jul, publisher = {acs}, doi = {10.1021/acsomega.1c03125}, url = {https://doi.org/10.1021/acsomega.1c03125}, dimensions = {true}, tab = {paper} } - Cover Picture
 SOMO–HOMO Conversion in Triplet CarbenesOrg. Lett., 2021, 23, 4955–4959.
SOMO–HOMO Conversion in Triplet CarbenesOrg. Lett., 2021, 23, 4955–4959.In this study, the SOMO–HOMO conversion has been shown for the first time in triplet carbenes embedded in cycloparaphenylene units. The high-lying HOMO originating from the curved π-conjugated system and the low-lying SOMO–1 originating due to the small carbene angle are the key to endowing this interesting electronic configuration. Furthermore, simple planar triplet carbenes such as fluorenylidene were found to possess SOMO–HOMO energy-converted electronic configurations.
@article{murata2021somo, title = {SOMO–HOMO Conversion in Triplet Carbenes}, author = {Murata, R. and Wang, Z. and Miyazawa, Y. and Antol, I. and Yamago, S. and Abe, M.}, journal = {Org. Lett.}, volume = {23}, issue = {13}, pages = {4955--4959}, year = {2021}, month = may, publisher = {acs}, doi = {10.1021/acs.orglett.1c01137}, url = {https://doi.org/10.1021/acs.orglett.1c01137}, dimensions = {true}, tab = {paper} } - Cover Picture
 1,3-Diradicals Embedded in Curved Paraphenylene Units: Singlet versus Triplet State and In-Plane AromaticityJ. Am. Chem. Soc., 2021, 143, 7426–7439.
1,3-Diradicals Embedded in Curved Paraphenylene Units: Singlet versus Triplet State and In-Plane AromaticityJ. Am. Chem. Soc., 2021, 143, 7426–7439.Curved π-conjugated molecules and open-shell structures have attracted much attention from the perspective of fundamental chemistry, as well as materials science. In this study, the chemistry of 1,3-diradicals (DRs) embedded in curved cycloparaphenylene (CPPs) structures, DR-(n+3)CPPs (n = 0–5), was investigated to understand the effects of the curvature and system size on the spin–spin interactions and singlet versus triplet state, as well as their unique characteristics such as in-plane aromaticity. A triplet ground state was predicted for the larger 1,3-diradicals, such as the seven- and eight-paraphenylene-unit-containing diradicals DR-7CPP (n = 4) and DR-8CPP (n = 5), by quantum chemical calculations. The smaller-sized diradicals DR-(n+3)CPPs (n = 0–3) were found to possess singlet ground states. Thus, the ground-state spin multiplicity is controlled by the size of the paraphenylene cycle. The size effect on the ground-state spin multiplicity was confirmed by the experimental generation of DR-6CPP in the photochemical denitrogenation of its azo-containing precursor (AZ-6CPP). Intriguingly, a unique type of in-plane aromaticity emerged in the smaller-sized singlet states such as S-DR-4CPP (n = 1), as proven by nucleus-independent chemical shift calculations (NICS) and an analysis of the anisotropy of the induced current density (ACID), which demonstrate that homoconjugation between the 1,3-diradical moiety arises because of the curved and distorted bonding system.
@article{miyazawa2021diradicals, title = {1,3-Diradicals Embedded in Curved Paraphenylene Units: Singlet versus Triplet State and In-Plane Aromaticity}, author = {Miyazawa, Y. and Wang, Z. and Matsumoto, M. and Hatano, S. and Antol, I. and Kayahara, E. and Yamago, S. and Abe, M.}, journal = {J. Am. Chem. Soc.}, volume = {143}, issue = {19}, pages = {7426--7439}, year = {2021}, month = apr, publisher = {acs}, doi = {10.1021/jacs.1c01329}, url = {https://doi.org/10.1021/jacs.1c01329}, dimensions = {true}, tab = {paper} } - HOT Article
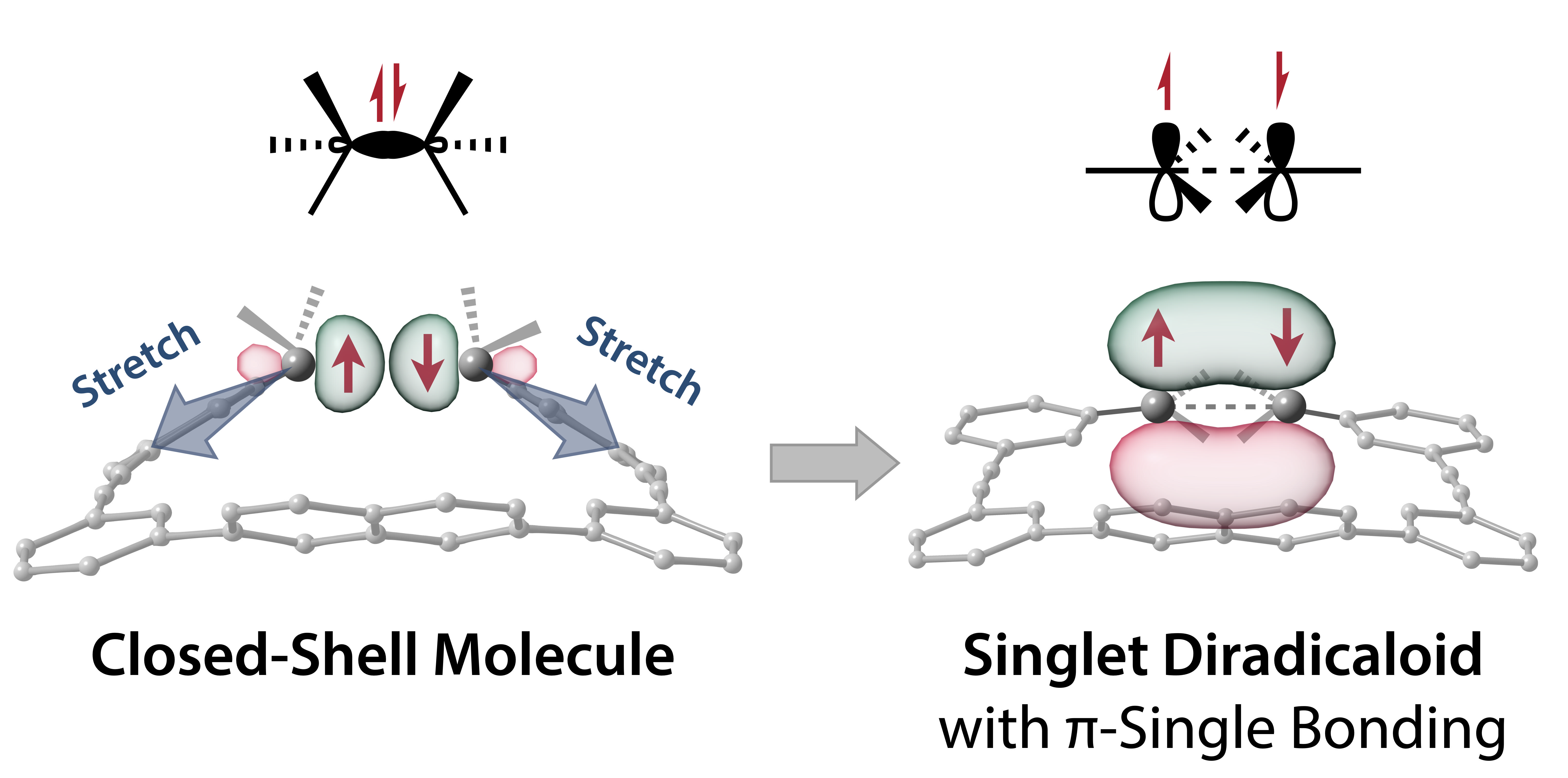 Impact of the macrocyclic structure and dynamic solvent effect on the reactivity of a localised singlet diradicaloid with π-single bonding characterZ. Wang, R. Akisaka, S. Yabumoto, T. Nakagawa, S. Hatano, and M. Abe*Chem. Sci., 2021, 12, 613–625.
Impact of the macrocyclic structure and dynamic solvent effect on the reactivity of a localised singlet diradicaloid with π-single bonding characterZ. Wang, R. Akisaka, S. Yabumoto, T. Nakagawa, S. Hatano, and M. Abe*Chem. Sci., 2021, 12, 613–625.Localised singlet diradicals are key intermediates in bond homolysis processes. Generally, these highly reactive species undergo radical–radical coupling reaction immediately after their generation. Therefore, their short-lived character hampers experimental investigations of their nature. In this study, we implemented the new concept of “stretch effect” to access a kinetically stabilised singlet diradicaloid. To this end, a macrocyclic structure was computationally designed to enable the experimental examination of a singlet diradicaloid with π-single bonding character. The kinetically stabilised diradicaloid exhibited a low carbon–carbon coupling reaction rate of 6.4 × 103 s−1 (155.9 μs), approximately 11 and 1000 times slower than those of the first generation of macrocyclic system (7.0 × 104 s−1, 14.2 μs) and the parent system lacking the macrocycle (5 × 106 s−1, 200 ns) at 293 K in benzene, respectively. In addition, a significant dynamic solvent effect was observed for the first time in intramolecular radical–radical coupling reactions in viscous solvents such as glycerin triacetate. This theoretical and experimental study demonstrates that the stretch effect and solvent viscosity play important roles in retarding the σ-bond formation process, thus enabling a thorough examination of the nature of the singlet diradicaloid and paving the way toward a deeper understanding of reactive intermediates.
@article{wang2021stretch, title = {Impact of the macrocyclic structure and dynamic solvent effect on the reactivity of a localised singlet diradicaloid with π-single bonding character}, author = {Wang, Z. and Akisaka, R. and Yabumoto, S. and Nakagawa, T. and Hatano, S. and Abe, M.}, journal = {Chem. Sci.}, volume = {12}, issue = {2}, pages = {613--625}, year = {2021}, month = jan, publisher = {rsc}, doi = {10.1039/d0sc05311b}, url = {https://doi.org/10.1039/d0sc05311b}, dimensions = {true}, tab = {paper} }
2018
-
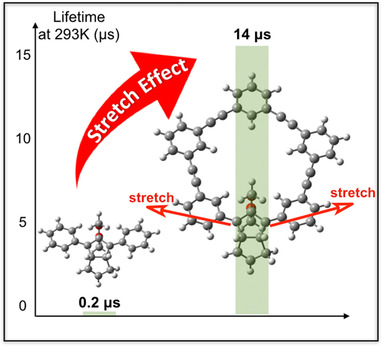 Extremely Long Lived Localized Singlet Diradicals in a Macrocyclic Structure: A Case Study on the Stretch EffectY. Harada, Z. Wang, S. Kumashiro, S. Hatano, and M. Abe*Chem. Eur. J., 2018, 24, 14808–14815.
Extremely Long Lived Localized Singlet Diradicals in a Macrocyclic Structure: A Case Study on the Stretch EffectY. Harada, Z. Wang, S. Kumashiro, S. Hatano, and M. Abe*Chem. Eur. J., 2018, 24, 14808–14815.Localized singlet diradicals have attracted much attention, not only in the field of bond-homolysis chemistry, but also in nonlinear optical materials. In this study, an extremely long lived localized singlet diradical was obtained by using a new molecular design strategy in which it is kinetically stabilized by means of a macrocycle that increases the molecular strain of the corresponding σ-bonded compound. Notably, the lifetime of this diradical (14 μs) is two orders of magnitude longer than that of a standard singlet diradical without a macrocyclic structure (≈0.2 μs) at 293 K. The species is persistent below a temperature of 100 K. In addition to the kinetic stabilization of the singlet diradical, the spontaneous oxidation of its corresponding ring-closed compound at 298 K produced oxygenated products under atmospheric conditions. Apparently, the “stretch effect” induced by the macrocyclic structure plays a crucial role in extending the lifetime of localized singlet diradicals and increasing the reactivity of their corresponding σ-bonded compounds.
@article{harada2018extremely, title = {Extremely Long Lived Localized Singlet Diradicals in a Macrocyclic Structure: A Case Study on the Stretch Effect}, author = {Harada, Y. and Wang, Z. and Kumashiro, S. and Hatano, S. and Abe, M.}, journal = {Chem. Eur. J.}, volume = {24}, issue = {55}, pages = {14808--14815}, year = {2018}, month = jul, publisher = {wiley}, doi = {10.1002/chem.201803076}, url = {https://doi.org/10.1002/chem.201803076}, dimensions = {true}, tab = {paper} }